Fevipiprant
Fevipiprant
Jump to navigation
Jump to search
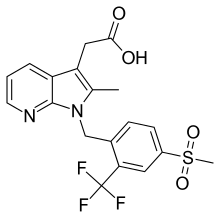 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
Pharmacokinetic data | |
| Bioavailability | Unaffected by food[1] |
| Metabolism | Hepatic glucuronidation |
| Elimination half-life | ~20 hours |
| Excretion | Renal (≤30%) |
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
PubChem CID |
|
| ChemSpider |
|
| UNII |
|
| KEGG |
|
| Chemical and physical data | |
| Formula | C19H17F3N2O4S |
| Molar mass | 426.41 g/mol |
| 3D model (JSmol) |
|
SMILES
| |
InChI
| |
Fevipiprant (INN; code name QAW039) is a drug being developed by Novartis which acts as a selective, orally available antagonist of the prostaglandin D2 receptor 2 (DP2 or CRTh2).[1][2][3]
As of 2016[update], it is in phase III[4]clinical trials for the treatment of asthma.[5]
See also[edit]
- Prostaglandin DP2 receptor
- Setipiprant
References[edit]
^ ab Erpenbeck VJ, Vets E, Gheyle L, Osuntokun W, Larbig M, Neelakantham S, et al. (2016). "Pharmacokinetics, Safety, and Tolerability of Fevipiprant (QAW039), a Novel CRTh2 Receptor Antagonist: Results From 2 Randomized, Phase 1, Placebo-Controlled Studies in Healthy Volunteers". Clin Pharmacol Drug Dev. 5 (4): 306–13. doi:10.1002/cpdd.244. PMC 5071756. PMID 27310331..mw-parser-output cite.citationfont-style:inherit.mw-parser-output qquotes:"""""""'""'".mw-parser-output code.cs1-codecolor:inherit;background:inherit;border:inherit;padding:inherit.mw-parser-output .cs1-lock-free abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-lock-limited a,.mw-parser-output .cs1-lock-registration abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-lock-subscription abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registrationcolor:#555.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration spanborder-bottom:1px dotted;cursor:help.mw-parser-output .cs1-hidden-errordisplay:none;font-size:100%.mw-parser-output .cs1-visible-errorfont-size:100%.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-formatfont-size:95%.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-leftpadding-left:0.2em.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-rightpadding-right:0.2em
^ Sykes DA, Bradley ME, Riddy DM, Willard E, Reilly J, Miah A, Bauer C, Watson SJ, Sandham DA, Dubois G, Charlton SJ.
Fevipiprant (QAW039), a Slowly Dissociating CRTh2 Antagonist with the Potential for Improved Clinical Efficacy. Mol Pharmacol. 2016 May;89(5):593-605. doi: 10.1124/mol.115.101832
PMID 26916831
^ Erpenbeck VJ, Popov TA, Miller D, Weinstein SF, Spector S, Magnusson B, et al. (2016). "The oral CRTh2 antagonist QAW039 (fevipiprant): A phase II study in uncontrolled allergic asthma". Pulm Pharmacol Ther. 39: 54–63. doi:10.1016/j.pupt.2016.06.005. PMID 27354118.
^ https://clinicaltrials.gov/ct2/show/NCT02555683
^ Gonem S, Berair R, Singapuri A, Hartley R, Laurencin M, Bacher G, et al. (2016). "Fevipiprant, a prostaglandin D2 receptor 2 antagonist, in patients with persistent eosinophilic asthma: a single-centre, randomised, double-blind, parallel-group, placebo-controlled trial". Lancet Respir Med. 4: 699–707. doi:10.1016/S2213-2600(16)30179-5.
This drug article relating to the respiratory system is a stub. You can help Wikipedia by expanding it. |
Categories:
- Drugs not assigned an ATC code
- Antiasthmatic drugs
- Receptor antagonists
- Sulfones
- Carboxylic acids
- Trifluoromethyl compounds
- Respiratory system drug stubs
(window.RLQ=window.RLQ||).push(function()mw.config.set("wgPageParseReport":"limitreport":"cputime":"0.708","walltime":"0.879","ppvisitednodes":"value":5021,"limit":1000000,"ppgeneratednodes":"value":0,"limit":1500000,"postexpandincludesize":"value":181862,"limit":2097152,"templateargumentsize":"value":5448,"limit":2097152,"expansiondepth":"value":14,"limit":40,"expensivefunctioncount":"value":4,"limit":500,"unstrip-depth":"value":1,"limit":20,"unstrip-size":"value":13059,"limit":5000000,"entityaccesscount":"value":3,"limit":400,"timingprofile":["100.00% 704.026 1 -total"," 58.46% 411.551 1 Template:Drugbox"," 38.70% 272.460 1 Template:Infobox"," 24.63% 173.407 1 Template:Reflist"," 19.58% 137.829 3 Template:Cite_journal"," 17.94% 126.329 8 Template:Navbox"," 12.65% 89.038 16 Template:Unbulleted_list"," 7.76% 54.636 1 Template:Prostanoidergics"," 6.22% 43.802 1 Template:Infobox_drug/chemical_formula"," 4.66% 32.795 21 Template:Abbr"],"scribunto":"limitreport-timeusage":"value":"0.271","limit":"10.000","limitreport-memusage":"value":4741778,"limit":52428800,"cachereport":"origin":"mw1270","timestamp":"20181215084507","ttl":1900800,"transientcontent":false);mw.config.set("wgBackendResponseTime":110,"wgHostname":"mw1274"););

 Clash Royale CLAN TAG
Clash Royale CLAN TAG