Prostaglandin D2
Prostaglandin D2
Jump to navigation
Jump to search
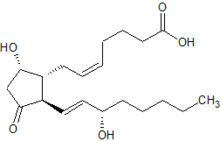 | |
| Names | |
|---|---|
IUPAC name 9α,15S-dihydroxy-11-oxo-prosta-5Z,13E-dien-1-oic acid | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
ChEBI |
|
ChemSpider |
|
ECHA InfoCard | 100.164.741 |
IUPHAR/BPS |
|
KEGG |
|
MeSH | Prostaglandin+D2 |
PubChem CID |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula | C20H32O5 |
Molar mass | 352.465 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Infobox references | |
Prostaglandin D2 (or PGD2) is a prostaglandin that binds to the receptor PTGDR (DP1), as well as CRTH2 (DP2).[1][2] It is a major prostaglandin produced by mast cells – recruits Th2 cells, eosinophils, and basophils. In mammalian organs, large amounts of PGD2 are found only in the brain and in mast cells. It is critical to development of allergic diseases such as asthma.
Research carried out in 1989[3] found PGD2 is the primary mediator of vasodilation (the "niacin flush") after ingestion of niacin (nicotinic acid).
A 2012 research paper indicates a causal link between elevated levels of localized PGD2 and hair growth inhibition.[4] Applied topically, the researchers found PGD2 prevents hair growth, and mice that were genetically inclined to produce higher levels of PGD2 had inhibited hair growth. The researchers also found PGD2 levels were much higher in balding scalp tissue than nonbalding scalp tissue, through increased levels of prostaglandin D2 synthase. The paper suggested that inhibition of hair growth involved binding of PGD2 to a receptor called GPR44, and that GPR44 therefore would be a therapeutic target for androgenic alopecia in both men and women with hair loss and thinning.[5] Because PGD2's relation to asthma has been known for several years, several drugs that seek to reduce the effect of PGD2 through blocking the GPR44 are already in clinical trials.[5]
Contents
1 Production
2 Effects
3 See also
4 References
Production[edit]
- Cellular synthesis occurs through the arachidonic acid cascade with the final conversion from PGH2 done by PGD2 synthase (PTGDS).
- In the brain, production occurs via an alternative pathway through the soluble, secreted enzyme β-trace [6]
Effects[edit]
- PGD2 causes a contraction of the bronchial airways. Its concentration in asthma patients is 10 times higher than in control patients, especially after it is brought into contact with allergens.
- It is involved in the regulation of reducing body temperature in sleep, and acts opposite to PGE2[citation needed].
- It causes vasoconstriction[7]
- Elevated levels of PGD2 and PGD2 synthase in scalp hair follicles may be partially responsible for male pattern baldness.[4]
- PGD2 also plays a part in male sexual development. It forms a feedforward loop with Sox9, which is activated by the SRY of the Y chromosome. PGD2, in a different feedforward loop than FGF9, helps keep the level of SOX9 high enough to activate other genes, such as Fgf9 and Sf1, which are necessary for the development of the male reproductive system [8]
- PGD2 plays a role in the attraction of neutrophils (chemotaxis).
See also[edit]
- PGD2 synthase
References[edit]
^ Saito S, Tsuda H, Michimata T (May 2002). "Prostaglandin D2 and reproduction". American Journal of Reproductive Immunology (New York, N.Y. : 1989). 47 (5): 295–302. doi:10.1034/j.1600-0897.2002.01113.x. PMID 12148545. Retrieved 2011-04-09..mw-parser-output cite.citationfont-style:inherit.mw-parser-output qquotes:"""""""'""'".mw-parser-output code.cs1-codecolor:inherit;background:inherit;border:inherit;padding:inherit.mw-parser-output .cs1-lock-free abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-lock-limited a,.mw-parser-output .cs1-lock-registration abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-lock-subscription abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registrationcolor:#555.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration spanborder-bottom:1px dotted;cursor:help.mw-parser-output .cs1-hidden-errordisplay:none;font-size:100%.mw-parser-output .cs1-visible-errorfont-size:100%.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-formatfont-size:95%.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-leftpadding-left:0.2em.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-rightpadding-right:0.2em
^ Pettipher R, Hansel TT (2008). "Antagonists of the prostaglandin D2 receptor CRTH2". Drug News & Perspectives. 21 (6): 317–22. doi:10.1358/dnp.2008.21.6.1246831. PMID 18836589. Retrieved 2011-04-09.
^ Morrow, JD; Parsons Wg, 3rd; Roberts Lj, 2nd (August 1989). "Release of markedly increased quantities of prostaglandin D2 in vivo in humans following the administration of nicotinic acid". Prostaglandins. 38 (2): 263–74. doi:10.1016/0090-6980(89)90088-9. PMID 2475889.
^ ab Garza, Luis A.,; et al. (2012-03-21). "Prostaglandin D2 Inhibits Hair Growth and Is Elevated in Bald Scalp of Men with Androgenetic Alopecia". Science Translational Medicine. 4 (126): 126ra34–126ra34. doi:10.1126/scitranslmed.3003122. ISSN 1946-6234. PMC 3319975. PMID 22440736. Retrieved 2012-03-22.
^ ab "Clues to the cause of male pattern baldness". Nature magazine (News and Comments).
^ Onoe, H.,; et al. (2012-05-21). "Prostaglandin D2, a cerebral sleep-inducing substance in monkeys". Proceedings of the National Academy of Sciences of the United States of America. 85 (11): 4082–4086. doi:10.1073/pnas.85.11.4082. PMC 280366. PMID 3163802.
^ https://www.clinicalkey.com/#!/browse/book/3-s2.0-C20140021375
^ Moniot, Brigitte; Declosmenil, Faustine; Barrionuevo, Francisco; Scherer, Gerd; Aritake, Kosuke; Malki, Safia; Marzi, Laetitia; Cohen-Solal, Ann; Georg, Ina; Klattig, Jürgen; Englert, Christoph; Kim, Yuna; Capel, Blanche; Eguchi, Naomi; Urade, Yoshihiro; Boizet-Bonhoure, Brigitte; Poulat, Francis (2009). "The PGD2 pathway, independently of FGF9, amplifies SOX9 activity in Sertoli cells during male sexual differentiation". Development. 136 (11): 1813–1821. doi:10.1242/dev.032631. PMC 4075598. PMID 19429785.
Categories:
- Prostaglandins
(window.RLQ=window.RLQ||).push(function()mw.config.set("wgPageParseReport":"limitreport":"cputime":"0.788","walltime":"0.984","ppvisitednodes":"value":5759,"limit":1000000,"ppgeneratednodes":"value":0,"limit":1500000,"postexpandincludesize":"value":223422,"limit":2097152,"templateargumentsize":"value":21634,"limit":2097152,"expansiondepth":"value":22,"limit":40,"expensivefunctioncount":"value":5,"limit":500,"unstrip-depth":"value":1,"limit":20,"unstrip-size":"value":24956,"limit":5000000,"entityaccesscount":"value":4,"limit":400,"timingprofile":["100.00% 805.922 1 -total"," 63.43% 511.215 1 Template:Chembox"," 42.62% 343.465 1 Template:Chembox_Identifiers"," 26.82% 216.145 3 Template:Chembox_headerbar"," 26.43% 213.037 9 Template:Trim"," 21.26% 171.359 11 Template:Navbox"," 18.11% 145.925 1 Template:Reflist"," 16.96% 136.708 11 Template:Main_other"," 14.80% 119.247 6 Template:Cite_journal"," 14.50% 116.823 1 Template:Chembox_parametercheck"],"scribunto":"limitreport-timeusage":"value":"0.338","limit":"10.000","limitreport-memusage":"value":6693063,"limit":52428800,"cachereport":"origin":"mw1263","timestamp":"20181215064612","ttl":1900800,"transientcontent":false););"@context":"https://schema.org","@type":"Article","name":"Prostaglandin D2","url":"https://en.wikipedia.org/wiki/Prostaglandin_D2","sameAs":"http://www.wikidata.org/entity/Q419750","mainEntity":"http://www.wikidata.org/entity/Q419750","author":"@type":"Organization","name":"Contributors to Wikimedia projects","publisher":"@type":"Organization","name":"Wikimedia Foundation, Inc.","logo":"@type":"ImageObject","url":"https://www.wikimedia.org/static/images/wmf-hor-googpub.png","datePublished":"2007-03-07T16:44:44Z","dateModified":"2018-08-18T13:29:01Z","image":"https://upload.wikimedia.org/wikipedia/commons/5/5e/Prostaglandin_D2_structure.png","headline":"chemical compound"(window.RLQ=window.RLQ||).push(function()mw.config.set("wgBackendResponseTime":122,"wgHostname":"mw1325"););

 Clash Royale CLAN TAG
Clash Royale CLAN TAG