Resveratrol
Resveratrol
Jump to navigation
Jump to search
 | |
 | |
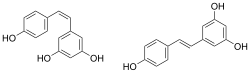 Chemical structures of cis- ((Z)-resveratrol, left) and trans-resveratrol ((E)-resveratrol, right)[1] | |
| Names | |
|---|---|
| Other names trans-3,5,4′-Trihydroxystilbene; 3,4′,5-Stilbenetriol; trans-Resveratrol; (E)-5-(p-Hydroxystyryl)resorcinol; (E)-5-(4-hydroxystyryl)benzene-1,3-diol | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
ChEBI |
|
ChEMBL |
|
ChemSpider |
|
DrugBank |
|
ECHA InfoCard | 100.121.386 |
KEGG |
|
PubChem CID |
|
RTECS number | CZ8987000 |
UNII |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula | C14H12O3 |
Molar mass | 228.25 g·mol−1 |
| Appearance | white powder with slight yellow cast |
Melting point | 261 to 263 °C (502 to 505 °F; 534 to 536 K)[2] |
Solubility in water | 0.03 g/L |
Solubility in DMSO | 16 g/L |
Solubility in ethanol | 50 g/L |
UV-vis (λmax) | 304nm (trans-resveratrol, in water) 286nm (cis-resveratrol, in water)[1] |
| Hazards | |
Safety data sheet | Fisher Scientific[2] Sigma Aldrich[3] |
R-phrases (outdated) | R36 (irritating to eyes)[3] |
S-phrases (outdated) | S26 (in case of contact with eyes, rinse immediately with plenty of water and seek medical advice)[3] |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) | 23.2 µM (5.29 g)[4] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Infobox references | |

UV visible spectrum of trans-resveratrol
Resveratrol (3,5,4′-trihydroxy-trans-stilbene) is a stilbenoid, a type of natural phenol, and a phytoalexin produced by several plants in response to injury or, when the plant is under attack by pathogens such as bacteria or fungi.[5][6] Sources of resveratrol in food include the skin of grapes, blueberries, raspberries, mulberries, and peanuts.[7][8]
Although it is used as a dietary supplement, there is no good evidence that consuming resveratrol affects life expectancy or human health.[9][10]
.mw-parser-output .toclimit-2 .toclevel-1 ul,.mw-parser-output .toclimit-3 .toclevel-2 ul,.mw-parser-output .toclimit-4 .toclevel-3 ul,.mw-parser-output .toclimit-5 .toclevel-4 ul,.mw-parser-output .toclimit-6 .toclevel-5 ul,.mw-parser-output .toclimit-7 .toclevel-6 uldisplay:none
Contents
1 Health effects
1.1 Heart disease
1.2 Cancer
1.3 Metabolism
1.4 Lifespan
2 Adverse effects
3 Pharmacology
3.1 Pharmacodynamics
3.2 Pharmacokinetics
3.2.1 Metabolism
4 Chemistry
4.1 Biosynthesis
4.2 Biotransformation
5 Occurrences
5.1 Plants
5.2 Foods
5.2.1 Wine and grape juice
5.2.2 Selected foods
5.3 Dietary supplements
6 History
7 Research
7.1 Cancer
7.2 Neurological studies
7.3 Cardiovascular studies
7.4 Diabetes
7.5 Skin
8 Related compounds
9 See also
10 References
11 External links
Health effects[edit]
Heart disease[edit]
There is no evidence of benefit from resveratrol in those who already have heart disease.[11] A 2014 Chinese meta-analysis found a statistically significant 11.90 mmHg reduction in systolic blood pressure from resveratrol doses of 150 mg/day.[12]
Cancer[edit]
As of 2014[update], there is no evidence of an effect of resveratrol on cancer in humans.[13]
Metabolism[edit]
There is no conclusive evidence for an effect of resveratrol on human metabolism.[14][15]
Lifespan[edit]
There is no evidence for an effect of resveratrol on lifespan in humans as of 2011[update].[16]
Adverse effects[edit]
A limited number of human studies have shown resveratrol is generally well-tolerated.[17][15]
A 2018 review of 17 clinical trials on the effects of resveratrol on blood pressure found that one person taking a 1000 mg daily dose developed an itchy rash that resolved after discontinuation, and that in four of the trials, people had increased frequency of bowel movements and loose stools in first month of the trial.[17] In a year long preliminary clinical trial in people with Alzheimer's disease, the most frequent adverse effects were diarrhea, weight loss, and nausea.[18]
Pharmacology[edit]
Pharmacodynamics[edit]
Resveratrol has been identified as a pan-assay interference compound, which produces positive results in many different laboratory assays.[19] Its ability for varied interactions may be due to direct effects on cell membranes.[20]
As of 2015, many specific biological targets for resveratrol had been identified or identified, including NQO2 (alone and in interaction with AKT1), GSTP1, estrogen receptor beta, CBR1, and integrin αVβ. It was unclear at that time if any or all of these were responsible for the observed effects in cells and model organisms.[21]
In vitro studies indicate resveratrol activates sirtuin 1,[22] although this may be a downstream effect from its immediate biological target(s).[23][24] It appears to signal through PGC-1α, thereby affecting mitochondria.[25] In cells treated with resveratrol, an increase is observed in the action of MnSOD (SOD2)[26] and in GPER activity.[27] In vitro, resveratrol was shown to act as an agonist of Peroxisome proliferator-activated receptor gamma, a nuclear receptor under pharmacological research as a potential treatment for type 2 diabetes.[28]
Pharmacokinetics[edit]
One way of administering resveratrol in humans may be buccal delivery by direct absorption through the saliva. However, the viability of a buccal delivery method is unlikely due to the low aqueous solubility of the molecule.[29][30] The bioavailability of resveratrol is about 0.5% due to extensive hepatic glucuronidation and sulfation.[31]
Metabolism[edit]
Resveratrol gets extensively metabolized in the body, with the liver and lungs as the major sites of its metabolism.[32]
Chemistry[edit]
Resveratrol (3,5,4'-trihydroxystilbene) is a stilbenoid, a derivative of stilbene.
It exists as two geometric isomers: cis- (Z) and trans- (E), with the trans-isomer shown in the top image. The trans- and cis-resveratrol can be either free or bound to glucose.[33]
The trans- form can undergo isomerization to the cis- form when exposed to ultraviolet irradiation,[34] a process called photoisomerization:[35]
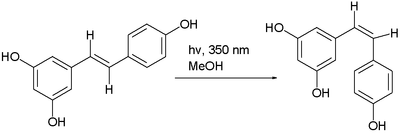
Resveratrol photoisomerization
One study showed that ultraviolet irradiation to cis-resveratrol induces further photochemical reaction, producing a fluorescent molecule named "Resveratrone".[36]
Trans-resveratrol in the powder form was found to be stable under "accelerated stability" conditions of 75% humidity and 40 °C in the presence of air.[37] The trans isomer is also stabilized by the presence of transport proteins.[38] Resveratrol content also was stable in the skins of grapes and pomace taken after fermentation and stored for a long period.[39]lH- and 13C-NMR data for the four most common forms of resveratrols are reported in literature.[33]
Biosynthesis[edit]
Resveratrol is produced in plants by the action of the enzyme, resveratrol synthase.[40]
Biotransformation[edit]
The grapevine fungal pathogen Botrytis cinerea is able to oxidise resveratrol into metabolites showing attenuated antifungal activities. Those include the resveratrol dimers restrytisol A, B, and C, resveratrol trans-dehydrodimer, leachinol F, and pallidol.[41] The soil bacterium Bacillus cereus can be used to transform resveratrol into piceid (resveratrol 3-O-beta-D-glucoside).[42]
Occurrences[edit]
Plants[edit]
Resveratrol is a phytoalexin, a class of compounds produced by many plants when they are infected by pathogens or physically harmed by cutting, crushing, or ultraviolet radiation.[43]
Plants that synthesize resveratrol include knotweeds, pine trees including Scots pine and Eastern white pine, grape vines, peanut plants, cocoa bushes, and Vaccinium shrubs that produce berries, including blueberries, raspberries, mulberries, cranberries, and bilberries.[5][7][43]
Foods[edit]
The levels of resveratrol found in food varies considerably, even in the same food from season to season and batch to batch.[5]
Wine and grape juice[edit]
| Beverage | Resveratrol (mg/150 ml)[8] | |
|---|---|---|
| mean | range | |
| Red wine | 0.27 | 0 — 2.78 |
| Rosé wine | 0.12 | 6997500000000000000♠5.00×10−03 — 0.29 |
| White wine | 0.04 | 0.00 — 0.17 |
| Sparkling wine | 6997900000000000000♠9.00×10−03 | 6997800000000000000♠8.00×10−03 — 6998100000000000000♠1.00×10−02 |
| Green grape juice | 6997508000000000000♠5.08×10−03 | 0.00 — 6998100000000000000♠1.00×10−02 |
In a 2007 review of published resveratrol concentrations, the average in red wines is 6994189999999999999♠1.9±1.7 mg trans-resveratrol/L (7000819999999999999♠8.2±7.5 µM, ranging from nondetectable levels to 14.3 mg/l (62.7 μM) trans-resveratrol. Levels of cis-resveratrol follow the same trend as trans-resveratrol.[44]
In general, wines made from grapes of the Pinot Noir and St. Laurent varieties showed the highest level of trans-resveratrol, though no wine or region can yet be said to produce wines with significantly higher concentrations than any other wine or region.[44]Champagne and vinegar also contain appreciable levels of resveratrol.[8]
Red wine contains between 0.2 and 5.8 mg/l, depending on the grape variety. White wine has much less because red wine is fermented with the skins, allowing the wine to extract the resveratrol, whereas white wine is fermented after the skin has been removed.[5] The composition of wine is different from that of grapes since the extraction of resveratrol from grapes depends on the duration of the skin contact, and the resveratrol 3-glucosides are in part hydrolysed, yielding both trans- and cis-resveratrol.[5]
Selected foods[edit]
| Food | Serving | Total resveratrol (mg)[5] |
|---|---|---|
| Peanuts (raw) | 1 cup (146 grams) | 0.01 – 0.26 |
| Peanut butter | 1 cup (258 grams) | 0.04 – 0.13 |
| Red grapes | 1 cup (160 grams) | 0.24 – 1.25 |
| Cocoa powder | 1 cup (200 grams) | 0.28 – 0.46 |
Ounce for ounce, peanuts have about 25% as much resveratrol as red wine.[5]Peanuts, especially sprouted peanuts, have a content similar to grapes in a range of 2.3 to 4.5 μg/g before sprouting, and after sprouting, in a range of 11.7 to 25.7 μg/g, depending upon peanut cultivar.[43][8]
Mulberries (especially the skin) are a source of as much as 50 micrograms of resveratrol per gram dry weight.[45]
Dietary supplements[edit]
Sales of resveratrol supplements increased in 2006 after studies on non-humans.[46]
Harvard University scientist and professor David Sinclair co-founded Sirtris Pharmaceuticals, the initial product of which was a resveratrol formulation;[47] Sinclair became known for making statements about resveratrol like: “(It's) as close to a miraculous molecule as you can find.... One hundred years from now, people will maybe be taking these molecules on a daily basis to prevent heart disease, stroke, and cancer.”[48] Most of the anti-aging field was more cautious, especially with regard to what else resveratrol might do in the body and its lack of bioavailability.[48][49]
Sinclair and others obtained significant news coverage about resveratrol.[50][51] Sinclair is often quoted and pictured in online ads for resveratrol supplements, many of which implied endorsement of the advertised product even though Sinclair had not endorsed them.[52]
History[edit]
The first mention of resveratrol was in a Japanese article in 1939 by Michio Takaoka, who isolated it from Veratrum album, variety grandiflorum, and later, in 1963, from the roots of Japanese knotweed.[43][53][54][55]
Research[edit]
A 2011 systematic review of existing resveratrol research demonstrated there was not enough evidence to demonstrate its effect on longevity or human diseases, nor could there be recommendations for intake beyond the amount normally obtained through dietary sources, estimated as being less than 4 mg/day.[9] Much of the research showing positive effects has been done on animals, with insufficient clinical research on humans.[9] Resveratrol research in animals and humans remains active.[56][57]
Cancer[edit]
As of 2014[update], the results of studies on laboratory animals or human clinical trials concerning the effects of resveratrol on cancer are inconsistent,[13] even if massive doses of resveratrol are used.[58]
Neurological studies[edit]
Resveratrol is under preliminary research for its potential to limit secondary damage after ischemic stroke or acute brain trauma,[59] and its possible effect on cognition.[18]
Cardiovascular studies[edit]
A 2018 meta-analysis found no effect on systolic or diastolic blood pressure; a sub-analysis revealed a 2 mmHg decrease in systolic pressure only from resveratrol doses of 300 mg per day, and only in diabetic people.[17] A 2015 meta-analysis found no effect on systolic or diastolic blood pressure; a sub-analysis found a 11.90 mmHg reduction in systolic blood pressure from resveratrol doses of 150 mg per day.[12]
Diabetes[edit]
One 2015 review found little evidence for use of resveratrol to treat diabetes.[60] A 2015 meta-analysis found little evidence for an effect of resveratrol on diabetes biomarkers.[61]
Skin[edit]
Despite considerable in vitro and animal research, there is no evidence that resveratrol taken orally or topically has any effect on human skin.[62] Preliminary studies have been conducted on resveratrol to understand its potential as a therapy for melanoma.[63][64]
Related compounds[edit]
- Dihydro-resveratrol
Epsilon-viniferin, Pallidol and Quadrangularin A three different resveratrol dimers
Trans-diptoindonesin B, a resveratrol trimer
Hopeaphenol, a resveratrol tetramer
Oxyresveratrol, the aglycone of mulberroside A, a compound found in Morus alba, the white mulberry[65]
Piceatannol, an active metabolite of resveratrol found in red wine
Piceid, a resveratrol glucoside
Pterostilbene, a doubly methylated resveratrol
4'-Methoxy-(E)-resveratrol 3-O-rutinoside, a compound found in the stem bark of Boswellia dalzielii[66]
Rhaponticin a glucoside of the stilbenoid rhapontigenin, found in rhubarb rhizomes
See also[edit]
- Phenolic compounds in wine
- Polyphenol antioxidant
- Wine and health
- List of phytochemicals in food
- Nutrition
- Phytochemistry
- Secondary metabolites
References[edit]
^ ab Camont L, Cottart CH, Rhayem Y, Nivet-Antoine V, Djelidi R, Collin F, Beaudeux JL, Bonnefont-Rousselot D; Cottart; Rhayem; Nivet-Antoine; Djelidi; Collin; Beaudeux; Bonnefont-Rousselot (February 2009). "Simple spectrophotometric assessment of the trans-/cis-resveratrol ratio in aqueous solutions". Anal. Chim. Acta. 634 (1): 121–8. doi:10.1016/j.aca.2008.12.003. PMID 19154820.CS1 maint: Multiple names: authors list (link) .mw-parser-output cite.citationfont-style:inherit.mw-parser-output qquotes:"""""""'""'".mw-parser-output code.cs1-codecolor:inherit;background:inherit;border:inherit;padding:inherit.mw-parser-output .cs1-lock-free abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-lock-limited a,.mw-parser-output .cs1-lock-registration abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-lock-subscription abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registrationcolor:#555.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration spanborder-bottom:1px dotted;cursor:help.mw-parser-output .cs1-hidden-errordisplay:none;font-size:100%.mw-parser-output .cs1-visible-errorfont-size:100%.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-formatfont-size:95%.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-leftpadding-left:0.2em.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-rightpadding-right:0.2em
^ ab Resveratrol MSDS on Fisher Scientific website
^ abc Resveratrol MSDS on www.sigmaaldrich.com
^ Bechmann LP, Zahn D, Gieseler RK, Fingas CD, Marquitan G, Jochum C, Gerken G, Friedman SL, Canbay A; Zahn; Gieseler; Fingas; Marquitan; Jochum; Gerken; Friedman; Canbay (June 2009). "Resveratrol amplifies profibrogenic effects of free fatty acids on human hepatic stellate cells". Hepatol. Res. 39 (6): 601–8. doi:10.1111/j.1872-034X.2008.00485.x. PMC 2893585. PMID 19207580.CS1 maint: Multiple names: authors list (link)
^ abcdefg Higdon J, Drake VJ, Steward WP (2016). "Resveratrol". Micronutrient Information Center. Linus Pauling Institute, Oregon State University, Corvallis, OR.
^ Fremont, Lucie (January 2000). "Biological Effects of Resveratrol". Life Sciences. 66 (8): 663–673. doi:10.1016/S0024-3205(99)00410-5. PMID 10680575. Retrieved 6 June 2014.
^ ab Jasiński M, Jasińska L, Ogrodowczyk M; Jasińska; Ogrodowczyk (August 2013). "Resveratrol in prostate diseases - a short review". Cent European J Urol. 66 (2): 144–9. doi:10.5173/ceju.2013.02.art8. PMC 3936154. PMID 24579014.CS1 maint: Multiple names: authors list (link)
^ abcd "Stilbenes-resveratrol in foods and beverages, version 3.6". Phenol-Explorer. 2016. Retrieved 13 May 2016.
^ abc Vang O, Ahmad N, Baile CA, Baur JA, Brown K, Csiszar A, et al. (2011). "What is new for an old molecule? Systematic review and recommendations on the use of resveratrol". PLoS ONE. 6 (6): e19881. Bibcode:2011PLoSO...619881V. doi:10.1371/journal.pone.0019881. PMC 3116821. PMID 21698226.
^ Sahebkar A, Serban C, Ursoniu S, Wong ND, Muntner P, Graham IM, Mikhailidis DP, Rizzo M, Rysz J, Sperling LS, Lip GY, Banach M (2015). "Lack of efficacy of resveratrol on C-reactive protein and selected cardiovascular risk factors--Results from a systematic review and meta-analysis of randomized controlled trials". Int. J. Cardiol. 189: 47–55. doi:10.1016/j.ijcard.2015.04.008. PMID 25885871.
^ Tomé-Carneiro J, Gonzálvez M, Larrosa M, Yáñez-Gascón MJ, García-Almagro FJ, Ruiz-Ros JA, Tomás-Barberán FA, García-Conesa MT, Espín JC; Gonzálvez; Larrosa; Yáñez-Gascón; García-Almagro; Ruiz-Ros; Tomás-Barberán; García-Conesa; Espín (Jul 2013). "Resveratrol in primary and secondary prevention of cardiovascular disease: a dietary and clinical perspective". Annals of the New York Academy of Sciences. 1290 (1): 37–51. Bibcode:2013NYASA1290...37T. doi:10.1111/nyas.12150. PMID 23855464.CS1 maint: Multiple names: authors list (link)
^ ab Liu Y, Ma W, Zhang P, He S, Huang D; Ma; Zhang; He; Huang (March 2014). "Effect of resveratrol on blood pressure: A meta-analysis of randomized controlled trials". Clinical Nutrition. 34 (1): 27–34. doi:10.1016/j.clnu.2014.03.009. PMID 24731650.CS1 maint: Multiple names: authors list (link)
^ ab Carter LG, D'Orazio JA, Pearson KJ; d'Orazio; Pearson (June 2014). "Resveratrol and cancer: focus on in vivo evidence". Endocr. Relat. Cancer. 21 (3): R209–25. doi:10.1530/ERC-13-0171. PMC 4013237. PMID 24500760.CS1 maint: Multiple names: authors list (link)
^ Poulsen MM, Jørgensen JO, Jessen N, Richelsen B, Pedersen SB; Jørgensen; Jessen; Richelsen; Pedersen (Jul 2013). "Resveratrol in metabolic health: an overview of the current evidence and perspectives". Annals of the New York Academy of Sciences. 1290 (1): 74–82. Bibcode:2013NYASA1290...74P. doi:10.1111/nyas.12141. PMID 23855468.CS1 maint: Multiple names: authors list (link)
^ ab Hausenblas HA, Schoulda JA, Smoliga JM; Schoulda; Smoliga (19 August 2014). "Resveratrol treatment as an adjunct to pharmacological management in type 2 diabetes mellitus-systematic review and meta-analysis". Molecular Nutrition & Food Research. 59 (1): 147–59. doi:10.1002/mnfr.201400173. PMID 25138371.CS1 maint: Multiple names: authors list (link)
^ Fernández AF, Fraga MF; Fraga (Jul 2011). "The effects of the dietary polyphenol resveratrol on human healthy aging and lifespan". Epigenetics. 6 (7): 870–4. doi:10.4161/epi.6.7.16499. PMID 21613817.
^ abc Fogacci F, Tocci G, Presta V, Fratter A, Borghi C, Cicero AFG (January 2018). "Effect of resveratrol on blood pressure: A systematic review and meta-analysis of randomized, controlled, clinical trials". Critical Reviews in Food Science and Nutrition. 58 (2): 1–14. doi:10.1080/10408398.2017.1422480. PMID 29359958.CS1 maint: Multiple names: authors list (link)
^ ab Ahmed, T; Javed, S; Javed, S; Tariq, A; Šamec, D; Tejada, S; Nabavi, SF; Braidy, N; Nabavi, SM (May 2017). "Resveratrol and Alzheimer's Disease: Mechanistic Insights". Molecular Neurobiology. 54 (4): 2622–2635. doi:10.1007/s12035-016-9839-9. PMID 26993301.
^ Baell, J; Walters, MA (25 September 2014). "Chemistry: Chemical con artists foil drug discovery". Nature. 513 (7519): 481–3. Bibcode:2014Natur.513..481B. doi:10.1038/513481a. PMID 25254460.
^ Ingólfsson, HI; Thakur, P; Herold, KF; Hobart, EA; Ramsey, NB; Periole, X; de Jong, DH; Zwama, M; Yilmaz, D; Hall, K; Maretzky, T; Hemmings HC, Jr; Blobel, C; Marrink, SJ; Koçer, A; Sack, JT; Andersen, OS (15 August 2014). "Phytochemicals perturb membranes and promiscuously alter protein function". ACS Chemical Biology. 9 (8): 1788–98. doi:10.1021/cb500086e. PMC 4136704. PMID 24901212.
^ Vang, O (August 2015). "Resveratrol: challenges in analyzing its biological effects". Annals of the New York Academy of Sciences. 1348 (1): 161–70. Bibcode:2015NYASA1348..161V. doi:10.1111/nyas.12879. PMID 26315294.
^ Alcaín FJ, Villalba JM; Villalba (April 2009). "Sirtuin activators". Expert Opin Ther Pat. 19 (4): 403–14. doi:10.1517/13543770902762893. PMID 19441923.
^ Beher D, Wu J, Cumine S, Kim KW, Lu SC, Atangan L, Wang M; Wu; Cumine; Kim; Lu; Atangan; Wang (December 2009). "Resveratrol is not a direct activator of SIRT1 enzyme activity". Chem Biol Drug des. 74 (6): 619–24. doi:10.1111/j.1747-0285.2009.00901.x. PMID 19843076.CS1 maint: Multiple names: authors list (link)
^ Pacholec M, Bleasdale JE, Chrunyk B, Cunningham D, Flynn D, Garofalo RS, Griffith D, Griffor M, Loulakis P, Pabst B, Qiu X, Stockman B, Thanabal V, Varghese A, Ward J, Withka J, Ahn K; Bleasdale; Chrunyk; Cunningham; Flynn; Garofalo; Griffith; Griffor; Loulakis; Pabst; Qiu; Stockman; Thanabal; Varghese; Ward; Withka; Ahn (March 2010). "SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1". J. Biol. Chem. 285 (11): 8340–51. doi:10.1074/jbc.M109.088682. PMC 2832984. PMID 20061378.CS1 maint: Multiple names: authors list (link)
^ Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J; Argmann; Gerhart-Hines; Meziane; Lerin; Daussin; Messadeq; Milne; Lambert; Elliott; Geny; Laakso; Puigserver; Auwerx (December 2006). "Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha". Cell. 127 (6): 1109–22. doi:10.1016/j.cell.2006.11.013. PMID 17112576.CS1 maint: Multiple names: authors list (link)
^ Macmillan-Crow LA, Cruthirds DL; Cruthirds (April 2001). "Invited review: manganese superoxide dismutase in disease". Free Radic. Res. 34 (4): 325–36. doi:10.1080/10715760100300281. PMID 11328670.
^ Prossnitz, Eric R.; Barton, Matthias (2014). "Estrogen biology: New insights into GPER function and clinical opportunities". Molecular and Cellular Endocrinology. 389 (1–2): 71–83. doi:10.1016/j.mce.2014.02.002. ISSN 0303-7207. PMC 4040308. PMID 24530924.
^ Wang L, Waltenberger B, Pferschy-Wenzig EM, Blunder M, Liu X, Malainer C, Blazevic T, Schwaiger S, Rollinger JM, Heiss EH, Schuster D, Kopp B, Bauer R, Stuppner H, Dirsch VM, Atanasov AG; Waltenberger; Pferschy-Wenzig; Blunder; Liu; Malainer; Blazevic; Schwaiger; Rollinger; Heiss; Schuster; Kopp; Bauer; Stuppner; Dirsch; Atanasov (2014). "Natural product agonists of peroxisome proliferator-activated receptor gamma (PPARγ): a review". Biochem Pharmacol. 92 (1): 73–89. doi:10.1016/j.bcp.2014.07.018. PMC 4212005. PMID 25083916.CS1 maint: Multiple names: authors list (link)
^ Madhav NV, Shakya AK, Shakya P, Singh K; Shakya; Shakya; Singh (November 2009). "Orotransmucosal drug delivery systems: a review". J Control Release. 140 (1): 2–11. doi:10.1016/j.jconrel.2009.07.016. PMID 19665039.CS1 maint: Multiple names: authors list (link)
^ Santos AC, Veiga F, Ribeiro AJ; Veiga; Ribeiro (August 2011). "New delivery systems to improve the bioavailability of resveratrol". Expert Opin Drug Deliv. 8 (8): 973–90. doi:10.1517/17425247.2011.581655. PMID 21668403.CS1 maint: Multiple names: authors list (link)
^ Walle T, Hsieh F, DeLegge MH, Oatis JE, Walle UK; Hsieh; Delegge; Oatis Jr; Walle (December 2004). "High absorption but very low bioavailability of oral resveratrol in humans". Drug Metab. Dispos. 32 (12): 1377–82. doi:10.1124/dmd.104.000885. PMID 15333514.CS1 maint: Multiple names: authors list (link)
^ Sharan S, Nagar S; Nagar (2013). "Pulmonary Metabolism of Resveratrol: In Vitro and in Vivo Evidence". Drug Metabolism and Disposition. 41 (5): 1163–9. doi:10.1124/dmd.113.051326. PMC 3629805. PMID 23474649.
^ ab Mattivi F, Reniero F, Korhammer S; Reniero; Korhammer (1995). "Isolation, characterization, and evolution in red wine vinification of resveratrol monomers". Journal of Agricultural and Food Chemistry. 43 (7): 1820–3. doi:10.1021/jf00055a013.CS1 maint: Multiple names: authors list (link)
^ Lamuela-Raventos RM, Romero-Perez AI, Waterhouse AL, de la Torre-Boronat MC; Romero-Perez; Waterhouse; de la Torre-Boronat (1995). "Direct HPLC Analysis of cis- and trans-Resveratrol and Piceid Isomers in Spanish Red Vitis vinifera Wines". Journal of Agricultural and Food Chemistry. 43 (2): 281–283. doi:10.1021/jf00050a003.CS1 maint: Multiple names: authors list (link)
^ Resveratrol Photoisomerization: An Integrative Guided-Inquiry Experiment Elyse Bernard, Philip Britz-McKibbin, Nicholas Gernigon Vol. 84 No. 7 July 2007 Journal of Chemical Education 1159.
^ Yang I, Kim E, Kang J, Han H, Sul S, Park SB, Kim SK; Kim; Kang; Han; Sul; Park; Kim (2012). "Photochemical generation of a new, highly fluorescent compound from non-fluorescent resveratrol". Chemical Communications. 48 (32): 3839–41. doi:10.1039/C2CC30940H. PMID 22436889.CS1 maint: Multiple names: authors list (link)
^ Prokop J, Abrman P, Seligson AL, Sovak M; Abrman; Seligson; Sovak (2006). "Resveratrol and its glycon piceid are stable polyphenols". J Med Food. 9 (1): 11–4. doi:10.1089/jmf.2006.9.11. PMID 16579722.CS1 maint: Multiple names: authors list (link)
^ Pantusa M, Bartucci R, Rizzuti B; Bartucci; Rizzuti (2014). "Stability of trans-resveratrol associated with transport proteins". J Agric Food Chem. 62 (19): 4384–91. doi:10.1021/jf405584a. PMID 24773207.CS1 maint: Multiple names: authors list (link)
^ Bertelli AA, Gozzini A, Stradi R, Stella S, Bertelli A; Gozzini; Stradi; Stella; Bertelli (1998). "Stability of resveratrol over time and in the various stages of grape transformation". Drugs Exp Clin Res. 24 (4): 207–11. PMID 10051967.CS1 maint: Multiple names: authors list (link)
^ Schröder G, Brown JW, Schröder J; Brown; Schröder (February 1988). "Molecular analysis of resveratrol synthase. cDNA, genomic clones and relationship with chalcone synthase". Eur. J. Biochem. 172 (1): 161–9. doi:10.1111/j.1432-1033.1988.tb13868.x. PMID 2450022.CS1 maint: Multiple names: authors list (link)
^ Cichewicz RH, Kouzi SA, Hamann MT; Kouzi; Hamann (January 2000). "Dimerization of resveratrol by the grapevine pathogen Botrytis cinerea". J. Nat. Prod. 63 (1): 29–33. doi:10.1021/np990266n. PMID 10650073.CS1 maint: Multiple names: authors list (link)
^ Cichewicz RH, Kouzi SA; Kouzi (October 1998). "Biotransformation of resveratrol to piceid by Bacillus cereus". J. Nat. Prod. 61 (10): 1313–4. doi:10.1021/np980139b. PMID 9784180.
^ abcd Sales, JM; Resurreccion, AV (2014). "Resveratrol in peanuts". Critical Reviews in Food Science and Nutrition. 54 (6): 734–70. doi:10.1080/10408398.2011.606928. PMID 24345046.
^ ab Stervbo U, Vang O, Bonnesen C; Vang; Bonnesen (2007). "A review of the content of the putative chemopreventive phytoalexin resveratrol in red wine". Food Chemistry. 101 (2): 449–57. doi:10.1016/j.foodchem.2006.01.047.CS1 maint: Multiple names: authors list (link)
^ Stewart JR, Artime MC, O'Brian CA; Artime; O'Brian (July 2003). "Resveratrol: a candidate nutritional substance for prostate cancer prevention". J. Nutr. 133 (7 Suppl): 2440S–2443S. doi:10.1093/jn/133.7.2440S. PMID 12840221.CS1 maint: Multiple names: authors list (link)
^ Seward ZM (2006-11-30). "Quest for youth drives craze for 'wine' pills". The Wall Street Journal.
^ McBride, Ryan (12 August 2010). "Former Sirtris Execs' Nonprofit Starts Selling Resveratrol with Potential Anti-Aging Effects Online". Xconomy.
^ ab Couzin, J (27 February 2004). "Scientific community. Aging research's family feud". Science. 303 (5662): 1276–9. doi:10.1126/science.303.5662.1276. PMID 14988530.
^ Wade, Nicholas (17 August 2009). "Tests Begin on Drugs That May Slow Aging". The New York Times.
^ Rimas A (2006-12-11). "His research targets the aging process". The Boston Globe.
^ Stipp D (2007-01-19). "Can red wine help you live forever?". Fortune magazine.
^ Weintraub A (2009-07-29). "Resveratrol: The Hard Sell on Anti-Aging". Bloomberg Businessweek.
^ Takaoka M (1939). "Resveratrol, a new phenolic compound, from Veratrum grandiflorum". Journal of the Chemical Society of Japan. 60 (11): 1090–1100. doi:10.1246/nikkashi1921.60.1090.
^ Takaoka, Michio (1940). "The Phenolic Substances of White Hellebore (Veratrum Grandiflorum Loes. Fill). V". Nippon Kagaku Kaishi. 61 (10): 1067–1069. doi:10.1246/nikkashi1921.61.1067.
^ Nonomura; Kanagawa (1963). "Chemical constituents of Polygonaceous plants. I. studies on the components of Ko-jo-kon. (Polygonum cuspidatum SIEB et ZUCC)". Yakugaku Zasshi. 83: 988–990.
^ Pangeni R, Sahni JK, Ali J, Sharma S, Baboota S; Sahni; Ali; Sharma; Baboota (2014). "Resveratrol: review on therapeutic potential and recent advances in drug delivery". Expert Opinion on Drug Delivery. 11 (8): 1285–1298. doi:10.1517/17425247.2014.919253. ISSN 1742-5247. PMID 24830814.CS1 maint: Multiple names: authors list (link)
^ Tomé-Carneiro J, Larrosa M, González-Sarrías A, Tomás-Barberán FA, García-Conesa MT, Espín JC; Larrosa; González-Sarrías; Tomás-Barberán; García-Conesa; Espín (2013). "Resveratrol and clinical trials: the crossroad from in vitro studies to human evidence". Curr. Pharm. Des. 19 (34): 6064–93. doi:10.2174/13816128113199990407. PMC 3782695. PMID 23448440.CS1 maint: Multiple names: authors list (link)
^ Athar M, Back JH, Tang X, Kim KH, Kopelovich L, Bickers DR, Kim AL; Back; Tang; Kim; Kopelovich; Bickers; Kim (November 2007). "Resveratrol: a review of preclinical studies for human cancer prevention". Toxicol. Appl. Pharmacol. 224 (3): 274–83. doi:10.1016/j.taap.2006.12.025. PMC 2083123. PMID 17306316.CS1 maint: Multiple names: authors list (link)
^ Lopez, M; Dempsey, R. J.; Vemuganti, R (2015). "Resveratrol Neuroprotection in Stroke and Traumatic CNS injury". Neurochemistry International. 89: 75–82. doi:10.1016/j.neuint.2015.08.009. PMC 4587342. PMID 26277384.
^ De Ligt, M; Timmers, S; Schrauwen, P (2015). "Resveratrol and obesity: Can resveratrol relieve metabolic disturbances?". Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 1852 (6): 1137–44. doi:10.1016/j.bbadis.2014.11.012. PMID 25446988.
^ Hausenblas HA, Schoulda JA, Smoliga JM (2015). "Resveratrol treatment as an adjunct to pharmacological management in type 2 diabetes mellitus--systematic review and meta-analysis". Mol Nutr Food Res. 59 (1): 147–59. doi:10.1002/mnfr.201400173. PMID 25138371.
^ Ndiaye, M; Philippe, C; Mukhtar, H; Ahmad, N (2011). "The Grape Antioxidant Resveratrol for Skin Disorders: Promise, Prospects, and Challenges". Archives of Biochemistry and Biophysics. 508 (2): 164–170. doi:10.1016/j.abb.2010.12.030. PMC 3060966. PMID 21215251.
^ Uzarska, M; Czajkowski, R; Schwartz, R. A.; Bajek, A; Zegarska, B; Drewa, T (2013). "Chemoprevention of skin melanoma: Facts and myths". Melanoma Research. 23 (6): 426–33. doi:10.1097/CMR.0000000000000016. PMID 24077511.
^ Pal, H. C.; Hunt, K. M.; Diamond, A; Elmets, C. A.; Afaq, F (2016). "Phytochemicals for the Management of Melanoma". Mini Reviews in Medicinal Chemistry. 16 (12): 953–79. doi:10.2174/1389557516666160211120157. PMC 4980238. PMID 26864554.
^ Kim JK, Kim M, Cho SG, Kim MK, Kim SW, Lim YH; Kim; Cho; Kim; Kim; Lim (June 2010). "Biotransformation of mulberroside A from Morus alba results in enhancement of tyrosinase inhibition". J. Ind. Microbiol. Biotechnol. 37 (6): 631–7. doi:10.1007/s10295-010-0722-9. PMID 20411402.CS1 maint: Multiple names: authors list (link)
^ Alemika Taiwo E, Onawunmi Grace O and Olugbade Tiwalade O, Antibacterial phenolics from Boswellia dalzielii. Nigerian Journal of Natural Products and Medicines, 2006
External links[edit]
 Media related to Resveratrol at Wikimedia Commons
Media related to Resveratrol at Wikimedia Commons
Categories:
- Resveratrol
- Aromatase inhibitors
- Phytoalexins
- Phytoestrogens
- Stilbenoids
(window.RLQ=window.RLQ||).push(function()mw.config.set("wgPageParseReport":"limitreport":"cputime":"1.876","walltime":"2.240","ppvisitednodes":"value":12185,"limit":1000000,"ppgeneratednodes":"value":0,"limit":1500000,"postexpandincludesize":"value":473255,"limit":2097152,"templateargumentsize":"value":30552,"limit":2097152,"expansiondepth":"value":21,"limit":40,"expensivefunctioncount":"value":8,"limit":500,"unstrip-depth":"value":1,"limit":20,"unstrip-size":"value":203946,"limit":5000000,"entityaccesscount":"value":5,"limit":400,"timingprofile":["100.00% 1722.682 1 -total"," 39.81% 685.782 1 Template:Reflist"," 37.17% 640.319 1 Template:Chembox"," 32.32% 556.699 54 Template:Cite_journal"," 21.59% 371.982 1 Template:Chembox_Identifiers"," 13.39% 230.744 16 Template:Navbox"," 13.36% 230.080 13 Template:Trim"," 13.32% 229.408 4 Template:Chembox_headerbar"," 9.62% 165.672 8 Template:Main_other"," 8.63% 148.617 1 Template:Chembox_parametercheck"],"scribunto":"limitreport-timeusage":"value":"0.869","limit":"10.000","limitreport-memusage":"value":10971996,"limit":52428800,"cachereport":"origin":"mw1256","timestamp":"20181227102103","ttl":1900800,"transientcontent":false);mw.config.set("wgBackendResponseTime":2368,"wgHostname":"mw1256"););

 Clash Royale CLAN TAG
Clash Royale CLAN TAG