Retinoid
Retinoid
Jump to navigation
Jump to search
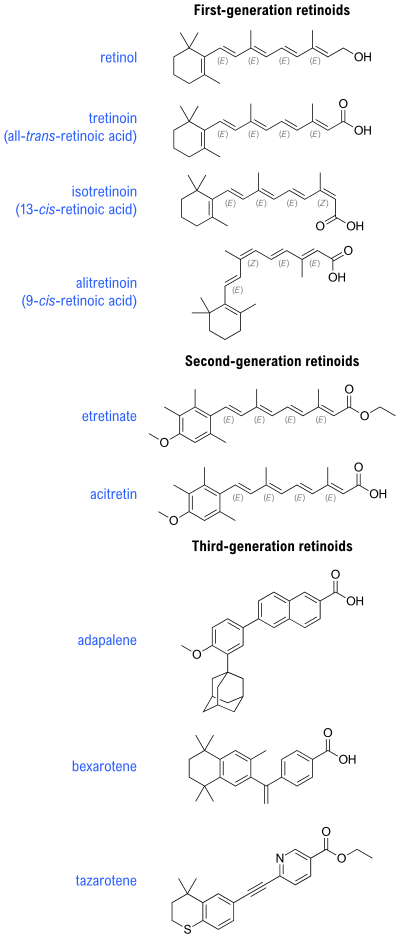
1st, 2nd, 3rd-generation retinoid compounds.
The retinoids are a class of chemical compounds that are vitamers of vitamin A or are chemically related to it. Retinoids have found use in medicine where they regulate epithelial cell growth.
Retinoids have many important functions throughout the body including roles in vision,[1] regulation of cell proliferation and differentiation, growth of bone tissue, immune function, and activation of tumor suppressor genes.
Research is also being done into their ability to treat skin cancers. Currently, alitretinoin (9-cis-retinoic acid) may be used topically to help treat skin lesions from Kaposi's sarcoma.
Contents
1 Types
2 Structure
3 Absorption
4 Uses
5 Synthesis
6 Toxicity
7 See also
8 References
9 External links
Types[edit]
There are three generations of retinoids:
- First generation include retinol, retinal, tretinoin (retinoic acid), isotretinoin, and alitretinoin
- Second generation include etretinate and its metabolite acitretin
- Third generation include adapalene, bexarotene, and tazarotene
Some authors consider retinoids derived from pyranones as the fourth-generation. One such compound is seletinoid G.[2]
Structure[edit]
The basic structure of the hydrophobic retinoid molecule consists of a cyclic end group, a polyene side chain and a polar end group. The conjugated system formed by alternating C=C double bonds in the polyene side chain are responsible for the color of retinoids (typically yellow, orange, or red). Hence, many retinoids are chromophores. Alternation of side chains and end groups creates the various classes of retinoids.
First- and second-generation retinoids are able to bind with several retinoid receptors due to the flexibility imparted by their alternating single and double bonds.
Third-generation retinoids are less flexible than first- and second-generation retinoids and therefore, interact with fewer retinoid receptors.
Absorption[edit]
The major source of retinoids from the diet are plant pigments such as carotenes and retinyl esters derived from animal sources. Retinyl esters are hydrolyzed in the intestinal lumen to yield free retinol and the corresponding fatty acid (i.e. palmitate or stearate). After hydrolysis, retinol is taken up by the enterocytes. Retinyl ester hydrolysis requires the presence of bile salts that serve to solubilize the retinyl esters in mixed micelles and to activate the hydrolyzing enzymes [3]
Several enzymes that are present in the intestinal lumen may be involved in the hydrolysis of dietary retinyl esters. Cholesterol esterase is secreted into the intestinal lumen from the pancreas and has been shown, in vitro, to display retinyl ester hydrolase activity. In addition, a retinyl ester hydrolase that is intrinsic to the brush-border membrane of the small intestine has been characterized in the rat as well as in the human. The different hydrolyzing enzymes are activated by different types of bile salts and have distinct substrate specificities. For example, whereas the pancreatic esterase is selective for short-chain retinyl esters, the brush-border membrane enzyme preferentially hydrolyzes retinyl esters containing a long-chain fatty acid such as palmitate or stearate. Retinol enters the absorptive cells of the small intestine, preferentially in the all-trans-retinol form.
Uses[edit]
Retinoids are used in the treatment of many diverse diseases and are effective in the treatment of a number of dermatological conditions such as inflammatory skin disorders,[citation needed]skin cancers,[citation needed] disorders of increased cell turnover (e.g. psoriasis),[4]photoaging,[5] and skin wrinkles [6]
Common skin conditions treated by retinoids include acne and psoriasis.[7]
Isotretinoin is not only considered the only known possible cure of acne in some patients, but was originally a chemotherapy treatment for certain cancers, such as leukemia.
Human embryonic stem cells also more readily differentiate into cortical stem cells in the presence of retinoids[8]
Retinoids are known to reduce the risk of head and neck cancers.[9][10]
Synthesis[edit]
Retinoids can be synthesized in a variety of ways. A common procedure to lower retinoid toxicity and improve retinoid activity is glucuronidation. Walker et al. have proposed a novel synthesis to accomplish glucuronidation.[11]
Toxicity[edit]
Toxic effects occur with prolonged high intake. The specific toxicity is related to exposure time and the exposure concentration. A medical sign of chronic poisoning is the presence of painful tender swellings on the long bones. Anorexia, skin lesions, hair loss, hepatosplenomegaly, papilloedema, bleeding, general malaise, pseudotumor cerebri, and death may also occur.[medical citation needed]
Chronic overdose also causes an increased lability of biological membranes and of the outer layer of the skin to peel.[12]
Recent research[citation needed] has suggested a role for retinoids in cutaneous adverse effects for a variety of drugs including the antimalarial drug proguanil. It is proposed that drugs such as proguanil act to disrupt retinoid homeostasis.
Systemic retinoids (isotretinoin, etretinate) are contraindicated during pregnancy as they may cause CNS, cranio-facial, cardiovascular and other defects.
See also[edit]
- Adapalene
- Hypervitaminosis A syndrome
- Biotinylated retinoids
References[edit]
^ Kiser, Philip D.; Golczak, Marcin; Palczewski, Krzysztof (11 July 2013). "Chemistry of the Retinoid (Visual) Cycle". Chemical Reviews. 114 (1): 194–232. doi:10.1021/cr400107q..mw-parser-output cite.citationfont-style:inherit.mw-parser-output qquotes:"""""""'""'".mw-parser-output code.cs1-codecolor:inherit;background:inherit;border:inherit;padding:inherit.mw-parser-output .cs1-lock-free abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-lock-limited a,.mw-parser-output .cs1-lock-registration abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-lock-subscription abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registrationcolor:#555.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration spanborder-bottom:1px dotted;cursor:help.mw-parser-output .cs1-hidden-errordisplay:none;font-size:100%.mw-parser-output .cs1-visible-errorfont-size:100%.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-formatfont-size:95%.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-leftpadding-left:0.2em.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-rightpadding-right:0.2em
^ Mukherjee, S; Date, A; Patravale, V; Korting, HC; Roeder, A; Weindl, G (2006). "Retinoids in the treatment of skin aging: an overview of clinical efficacy and safety". Clinical Interventions in Aging. 1 (4): 327–48. doi:10.2147/ciia.2006.1.4.327. PMC 2699641. PMID 18046911.
^ Noy, N. (2006) "Vitamin A", "Biochemical, Physiological, & Molecular Aspects of Human Nutrition", M. H. Stipanuk 2nd Ed.
^ "Psoriasis - Diagnosis and treatment - Mayo Clinic". www.mayoclinic.org. Archived from the original on 7 May 2017. Retrieved 16 March 2018.
^ Stefanaki C, Stratigos A, Katsambas A (June 2005). "Topical retinoids in the treatment of photoaging". J Cosmet Dermatol. 4 (2): 130–4. doi:10.1111/j.1473-2165.2005.40215.x. PMID 17166212.
^ Kafi, R; Kwak, HS; Schumacher, WE; Cho, S; Hanft, VN; Hamilton, TA; King, AL; Neal, JD; Varani, J; Fisher, GJ; Voorhees, JJ; Kang, S (2007). "Improvement of naturally aged skin with vitamin A (retinol)". Arch Dermatol. 143: 606–12. doi:10.1001/archderm.143.5.606. PMID 17515510.
^ "National Psoriasis Foundation". psoriasis.org. Archived from the original on 13 June 2010. Retrieved 16 March 2018.
^ http://www.nature.com/neuro/journal/vaop/ncurrent/fig_tab/nn.3041_F1.html Archived November 5, 2014, at the Wayback Machine.
^ Smith, W; Saba, N (2005). "Retinoids as chemoprevention for head and neck cancer: Where do we go from here?". Critical reviews in oncology/hematology. 55 (2): 143–52. doi:10.1016/j.critrevonc.2005.02.003. PMID 15886010.
^ 2009 Internal Medicine Boards-Style Q&A Archived 2012-09-19 at the Wayback Machine., Q:378, p.232; A:378, p.125
^ Walker, JR; Alshafie, G; Abou-Issa, H; Curley, RW (12 September 2002). "An Improved Synthesis of the C-linked Glucuronide of N-(4-Hydroxyphenyl)retinamide". Bioorganic & Medicinal Chemistry Letters. 12 (17): 2447–50. doi:10.1016/s0960-894x(02)00427-4. PMID 12161154.
^ "Topical retinoids - DermNet New Zealand". dermnetnz.org. Archived from the original on 29 July 2016. Retrieved 16 March 2018.
The Pharmacological Basis of Therapeutics, 10th Edition, Goodman & Gilman.
Clinical Pharmacology, P.N. Bennett & M.J. Brown.
External links[edit]
Retinoids at the US National Library of Medicine Medical Subject Headings (MeSH)
Categories:
- Retinoids
(window.RLQ=window.RLQ||).push(function()mw.config.set("wgPageParseReport":"limitreport":"cputime":"0.608","walltime":"0.732","ppvisitednodes":"value":2474,"limit":1000000,"ppgeneratednodes":"value":0,"limit":1500000,"postexpandincludesize":"value":180492,"limit":2097152,"templateargumentsize":"value":4117,"limit":2097152,"expansiondepth":"value":13,"limit":40,"expensivefunctioncount":"value":5,"limit":500,"unstrip-depth":"value":1,"limit":20,"unstrip-size":"value":28671,"limit":5000000,"entityaccesscount":"value":2,"limit":400,"timingprofile":["100.00% 441.449 1 -total"," 35.09% 154.895 11 Template:Navbox"," 30.81% 136.017 6 Template:Cite_journal"," 22.99% 101.501 3 Template:Citation_needed"," 19.96% 88.109 4 Template:Fix"," 11.97% 52.829 1 Template:Prostanoid_signaling_modulators"," 10.83% 47.796 7 Template:Category_handler"," 7.65% 33.777 4 Template:Delink"," 7.15% 31.581 20 Template:Abbrlink"," 6.66% 29.401 22 Template:Abbr"],"scribunto":"limitreport-timeusage":"value":"0.233","limit":"10.000","limitreport-memusage":"value":4939947,"limit":52428800,"cachereport":"origin":"mw1241","timestamp":"20181227184339","ttl":1900800,"transientcontent":false);mw.config.set("wgBackendResponseTime":98,"wgHostname":"mw1275"););

 Clash Royale CLAN TAG
Clash Royale CLAN TAG