Selenium tetrachloride
 |
 |
Names
|
|---|
IUPAC name
Selenium tetrachloride
|
Identifiers
|
|---|
CAS Number
|
10026-03-6  N N
|
3D model (JSmol)
|
|
ChemSpider
|
59590  Y Y
|
ECHA InfoCard
|
100.030.036
|
|
|
RTECS number
|
VS7875000
|
UNII
|
4GB8868P5J  N N
|
InChI InChI=1S/Cl4Se/c1-5(2,3)4  Y YKey: LNBXMNQCXXEHFT-UHFFFAOYSA-N  Y Y
InChI=1/Cl4Se/c1-5(2,3)4 Key: LNBXMNQCXXEHFT-UHFFFAOYAD
|
|
Properties
|
|---|
Chemical formula
|
SeCl4 |
Molar mass
|
220.771 g/mol
|
Appearance
|
white to yellow crystals
|
Density
|
2.6 g/cm3, solid
|
Melting point
|
sublimes at 191.4 °C[1] |
Solubility in water
|
decomposes in water
|
Structure
|
|---|
Crystal structure
|
Monoclinic, mS80
|
Space group
|
C12/c1, No. 15
|
Molecular shape
|
Seesaw (gas phase)[citation needed] |
Hazards
|
|---|
EU classification (DSD) (outdated)
|
 T T  N N
|
R-phrases (outdated)
|
R23/25, R33, R50/53
|
S-phrases (outdated)
|
S20/21, S28, S45, S60, S61[2] |
NFPA 704
|
Flash point
|
non-flammable
|
Related compounds
|
|---|
Other anions
|
Selenium tetrafluoride
Selenium tetrabromide
Selenium dioxide
|
Other cations
|
Dichlorine monoxide
Sulfur tetrachloride
Tellurium tetrachloride
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
 N verify (what is N verify (what is  Y Y N ?) N ?)
|
Infobox references
|
|
|
Selenium tetrachloride is the inorganic compound composed with the formula SeCl4. This compound exists as yellow to white volatile solid. It is one of two commonly available selenium chlorides, the other example being selenium monochloride, Se2Cl2. SeCl4 is used in the synthesis of other selenium compounds.
Synthesis and structure[edit]
The compound is prepared by treating selenium with chlorine.[3] When the reacting selenium is heated, the product sublimes from the reaction flask. The volatility of selenium tetrachloride can be exploited to purification of selenium.
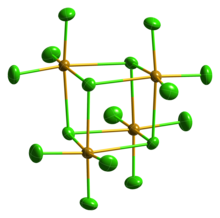
Solid SeCl4 is actually a tetrameric cubane-type cluster, for which the Se atom of an SeCl6 octahedron sits on four corners of the cube and the bridging Cl atoms sit on the other four corners. The bridging Se-Cl distances are longer than the terminal Se-Cl distances, but all Cl-Se-Cl angles are approximately 90°.[4]
SeCl4 has often been used as an example for teaching VSEPR rules of hypervalent molecules. As such, one would predict four bonds but five electron groups giving rise to a seesaw geometry. This clearly is not the case in the crystal structure. Others have suggested that the crystal structure can be represented as SeCl3+ and Cl−. This formulation would predict a pyramidal geometry for the SeCl3+ cation with a Cl-Se-Cl bond angle of approximately 109°. However, this molecule is an excellent example of a situation where maximal bonding cannot be achieved with the simplest molecular formula. The formation of the tetramer (SeCl4)4,[5] with delocalized sigma bonding of the bridging chloride is clearly preferred over a "hypervalent" small molecule.
Gaseous SeCl4 contains SeCl2 and chlorine, which recombine upon condensation.
Reactions[edit]
Selenium tetrachloride can be reduced in situ to the dichloride using triphenylstibine:
- SeCl4 + SbPh3 → SeCl2 + Cl2SbPh3
Selenium tetrachloride reacts with water to give selenous and hydrochloric acids:[6][page needed]
- SeCl4 + 3 H2O → H2SeO3 + 4 HCl
Upon treatment with selenium dioxide, it gives selenium oxychloride:[6][page needed]
- SeCl4 + SeO2 → 2SeOCl2
References[edit]
^
Lide, David R. (1998). Handbook of Chemistry and Physics (87 ed.). Boca Raton, Florida: CRC Press. p. 487. ISBN 0-8493-0594-2. Retrieved 2008-07-02..mw-parser-output cite.citationfont-style:inherit.mw-parser-output qquotes:"""""""'""'".mw-parser-output code.cs1-codecolor:inherit;background:inherit;border:inherit;padding:inherit.mw-parser-output .cs1-lock-free abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-lock-limited a,.mw-parser-output .cs1-lock-registration abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-lock-subscription abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registrationcolor:#555.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration spanborder-bottom:1px dotted;cursor:help.mw-parser-output .cs1-hidden-errordisplay:none;font-size:100%.mw-parser-output .cs1-visible-errorfont-size:100%.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-formatfont-size:95%.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-leftpadding-left:0.2em.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-rightpadding-right:0.2em
^
"323527 Selenium tetrachloride". Sigma-Aldrich. Retrieved 2008-07-02.
^ Nowak, H. G.; Suttle, J. F.; Parker, W. E.; Kleinberg, J. (1957). "Selenium (IV) Chloride". Inorganic Syntheses. Inorganic Syntheses. 5. p. 125. doi:10.1002/9780470132364.ch33. ISBN 9780470132364.
^ Kristallstruktur der stabilen Modifikation von SeCl4, Zeitschrift fur Naturforschung, 36b, 1660, 1981
^ Wells, Structural Inorganic Chemistry, fifth ed, Oxford, p. 709,
ISBN 0-19-855370-6
^ ab Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 0-08-037941-9.
Selenium compounds
|
|---|
| Se(0,I) |
|
|---|
| Se(I) |
|
|---|
| Se(II) |
Selenides
|
|---|
H2Se
H2Se2
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
He
|
Li2Se
|
Be
|
|
|
|
|
|
|
|
|
|
|
B
|
CSe2
COSe
|
(NH4)2Se
|
O
|
F
|
Ne
|
Na2Se
|
MgSe
|
|
|
|
|
|
|
|
|
|
|
Al2Se3
|
Si
|
PxSey
|
S
|
Cl
|
Ar
|
K2Se
|
CaSe
|
Sc2Se3
|
TiSe2
|
V
|
CrSe
Cr2Se3
|
MnSe
MnSe2
|
FeSe
|
CoSe
|
NiSe
|
CuSe
|
ZnSe
|
GaSe
Ga2Se3
|
GeSe
|
As2Se3
As4Se3
|
Se
|
Br
|
Kr
|
Rb2Se
|
SrSe
|
Y2Se3
|
Zr
|
NbSe2
|
MoSe2
|
Tc
|
Ru
|
Rh
|
Pd
|
Ag2Se
|
CdSe
|
In2Se3
|
SnSe
SnSe2
|
Sb2Se3
|
Te
|
I
|
Xe
|
Cs2Se
|
BaSe
|
*
|
Hf
|
Ta
|
WSe2
WSe3
|
ReSe2
|
Os
|
Ir
|
Pt
|
Au
|
HgSe
|
Tl2Se
|
PbSe
|
Bi2Se3
|
Po
|
At
|
Rn
|
Fr
|
Ra
|
**
|
Rf
|
Db
|
Sg
|
Bh
|
Hs
|
Mt
|
Ds
|
Rg
|
Cn
|
Nh
|
Fl
|
Mc
|
Lv
|
Ts
|
Og
|
|
|
|
|
|
|
|
|
|
|
↓
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
*
|
La2Se3
|
Ce2Se3
|
Pr2Se3
|
Nd2Se3
|
Pm
|
Sm2Se3
|
Eu2Se3
|
Gd
|
Tb
|
Dy
|
Ho
|
Er2Se3
|
Tm2Se3
|
Yb
|
Lu
|
|
|
**
|
Ac
|
ThSe2
|
Pa
|
USe2
|
Np
|
Pu
|
Am
|
Cm
|
Bk
|
Cf
|
Es
|
Fm
|
Md
|
No
|
Lr
|
|
|
|
|---|
| Se(IV) |
- SeCl4
- SeF4
- SeO2
- SeS2
- SeOBr2
- SeOCl2
|
|---|
| Se(VI) |
|
|---|
Prostanoid signaling modulators
|
|---|
Receptor
(ligands)
|
|
|---|
Enzyme
(inhibitors)
|
COX
(PTGS) |
|
|---|
| PGD2S |
- Retinoids
Selenium (selenium tetrachloride, sodium selenite, selenium disulfide)
|
|---|
| PGES |
HQL-79 |
|---|
| PGFS |
Bimatoprost |
|---|
| PGI2S |
Tranylcypromine |
|---|
| TXAS |
- Camonagrel
- Dazmegrel
- Dazoxiben
- Furegrelate
- Isbogrel
- Midazogrel
- Nafagrel
- Nicogrelate
- Ozagrel
- Picotamide
- Pirmagrel
- Ridogrel
- Rolafagrel
- Samixogrel
- Terbogrel
- U63557A
|
|---|
|
|---|
| Others |
Precursors: Linoleic acid
- γ-Linolenic acid (gamolenic acid)
- Dihomo-γ-linolenic acid
- Diacylglycerol
- Arachidonic acid
- Prostaglandin G2
- Prostaglandin H2
|
|---|
- See also
- Receptor/signaling modulators
- Leukotriene signaling modulators
- Nuclear receptor modulators
|
|

Categories:
- Selenium compounds
- Chlorides
- Nonmetal halides
- Chalcohalides
Hidden categories:
- Articles without EBI source
- Articles without KEGG source
- Articles with changed CASNo identifier
- ECHA InfoCard ID from Wikidata
- Articles with changed FDA identifier
- All articles with unsourced statements
- Articles with unsourced statements from February 2012
- Chembox having DSD data
- Articles containing unverified chemical infoboxes
- Wikipedia articles needing page number citations from April 2013
Navigation menu
Personal tools
- Not logged in
- Talk
- Contributions
- Create account
- Log in
Navigation
- Main page
- Contents
- Featured content
- Current events
- Random article
- Donate to Wikipedia
- Wikipedia store
Interaction
- Help
- About Wikipedia
- Community portal
- Recent changes
- Contact page
Tools
- What links here
- Related changes
- Upload file
- Special pages
- Permanent link
- Page information
- Wikidata item
- Cite this page
Print/export
- Create a book
- Download as PDF
- Printable version
Languages
- تۆرکجه
- Deutsch
- Español
- فارسی
- Nederlands
- Português
- Русский
- Simple English
- Српски / srpski
- Srpskohrvatski / српскохрватски
Edit links
(window.RLQ=window.RLQ||).push(function()mw.config.set("wgPageParseReport":"limitreport":"cputime":"0.772","walltime":"1.059","ppvisitednodes":"value":7446,"limit":1000000,"ppgeneratednodes":"value":0,"limit":1500000,"postexpandincludesize":"value":314278,"limit":2097152,"templateargumentsize":"value":37535,"limit":2097152,"expansiondepth":"value":22,"limit":40,"expensivefunctioncount":"value":4,"limit":500,"unstrip-depth":"value":1,"limit":20,"unstrip-size":"value":15431,"limit":5000000,"entityaccesscount":"value":2,"limit":400,"timingprofile":["100.00% 857.435 1 -total"," 70.27% 602.480 1 Template:Chembox"," 33.51% 287.287 1 Template:Chembox_Identifiers"," 21.51% 184.395 6 Template:Chembox_headerbar"," 21.11% 180.991 20 Template:Trim"," 17.27% 148.100 1 Template:Reflist"," 13.82% 118.510 7 Template:Navbox"," 13.03% 111.724 12 Template:Main_other"," 11.06% 94.810 1 Template:Chembox_parametercheck"," 9.69% 83.094 3 Template:Cite_book"],"scribunto":"limitreport-timeusage":"value":"0.273","limit":"10.000","limitreport-memusage":"value":5353129,"limit":52428800,"cachereport":"origin":"mw1264","timestamp":"20181217065934","ttl":1900800,"transientcontent":false);mw.config.set("wgBackendResponseTime":97,"wgHostname":"mw1258"););



 Clash Royale CLAN TAG
Clash Royale CLAN TAG